One for Multiple: Physics-Informed Synthetic Data Boosts Generalizable Deep Learning for Fast MRI Reconstruction(中文,English)
Zi Wang1,2, Xiaotong Yu1, Chengyan Wang3,4, Weibo Chen5, Jiazheng Wang5, Ying-Hua Chu6, Hongwei Sun7, Rushuai Li8, Peiyong Li9, Fan Yang10, Haiwei Han10, Taishan Kang11, Jianzhong Lin11, Chen Yang12, Shufu Chang13, Zhang Shi14, Sha Hua15, Yan Li16, Juan Hu17, Liuhong Zhu18, Jianjun Zhou18, Meijing Lin19, Jiefeng Guo20, Congbo Cai1, Zhong Chen1, Di Guo21, Guang Yang 2,22,23,24, Xiaobo Qu1,*
1 Department of Electronic Science, Xiamen University-Neusoft Medical Magnetic Resonance Imaging Joint Research and Development Center, Fujian Provincial Key Laboratory of Plasma and Magnetic Resonance, National Institute for Data Science in Health and Medicine, Xiamen University, China.
2Department of Bioengineering and Imperial-X, Imperial College London, United Kingdom.
3 Shanghai Pudong Hospital and Human Phenome Institute, Fudan University, China.
4 International Human Phenome Institute (Shanghai), China.
5 Philips Healthcare, China.
6 Siemens Healthineers Ltd., China.
7 United Imaging Research Institute of Intelligent Imaging, China.
8 Department of Nuclear Medicine, Nanjing First Hospital, China.
9 Shandong Aoxin Medical Technology Company, China.
10 Department of Radiology, The First Affiliated Hospital of Xiamen University, China.
11 Department of Radiology, Zhongshan Hospital Affiliated to Xiamen University, China.
12 Department of Neurosurgery, Zhongshan Hospital, Fudan University (Xiamen Branch), China.
13 Department of Cardiology, Shanghai Institute of Cardiovascular Diseases, Zhongshan Hospital, Fudan University, China.
14 Department of Radiology, Zhongshan Hospital, Fudan University, China.
15 Department of Cardiovascular Medicine, Heart Failure Center, Ruijin Hospital Lu Wan Branch, Shanghai Jiaotong University School of Medicine, China.
16 Department of Radiology, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, China.
17 Medical Imaging Department, The First Affiliated Hospital of Kunming Medical University, China.
18 Department of Radiology, Zhongshan Hospital, Fudan University (Xiamen Branch), China.
19 Department of Applied Marine Physics and Engineering, Xiamen University, China.
20 Department of Microelectronics and Integrated Circuit, Xiamen University, China.
21 School of Computer and Information Engineering, Xiamen University of Technology, China.
22 National Heart and Lung Institute, Imperial College London, United Kingdom.
23 Cardiovascular Research Centre, Royal Brompton Hospital, United Kingdom.
24 School of Biomedical Engineering & Imaging Sciences, King’s College London, United Kingdom.
* Emails: quxiaobo <at> xmu.edu.cn
Citation
Zi Wang, Xiaotong Yu, Chengyan Wang, Weibo Chen, Jiazheng Wang, Ying-Hua Chu, Hongwei Sun, Rushuai Li, Peiyong Li, Fan Yang, Haiwei Han, Taishan Kang, Jianzhong Lin, Chen Yang, Shufu Chang, Zhang Shi, Sha Hua, Yan Li, Juan Hu, Liuhong Zhu, Jianjun Zhou, Meijing Lin, Jiefeng Guo, Congbo Cai, Zhong Chen, Di Guo, Guang Yang, Xiaobo Qu, One for multiple: Physics-informed synthetic data boosts generalizable deep learning for fast MRI reconstruction, Medical Image Analysis, 103: 103616, 2025.
Synopsis
Magnetic resonance imaging (MRI) is a widely used radiological modality renowned for its radiation-free, comprehensive insights into the human body, facilitating medical diagnoses. However, the drawback of prolonged scan times hinders its accessibility. The k-space undersampling offers a solution, yet the resultant artifacts necessitate meticulous removal during image reconstruction. Although deep learning (DL) has proven effective for fast MRI image reconstruction, its broader applicability across various imaging scenarios has been constrained. Challenges include the high cost and privacy restrictions associated with acquiring large-scale, diverse training data, coupled with the inherent difficulty of addressing mismatches between training and target data in existing DL methodologies. Here, we present a novel Physics-Informed Synthetic data learning Framework for fast MRI, called PISF. PISF marks a breakthrough by enabling generalizable DL for multi-scenario MRI reconstruction through a single trained model. Our approach separates the reconstruction of a 2D image into many 1D basic problems, commencing with 1D data synthesis to facilitate generalization. We demonstrate that training DL models on synthetic data, coupled with enhanced learning techniques, yields in vivo MRI reconstructions comparable to or surpassing those of models trained on matched realistic datasets, reducing the reliance on real-world MRI data by up to 96 %. With a single trained model, our PISF supports the high-quality reconstruction under 4 sampling patterns, 5 anatomies, 6 contrasts, 5 vendors, and 7 centers, exhibiting remarkable generalizability. Its adaptability to 2 neuro and 2 cardiovascular patient populations has been validated through evaluations by 10 experienced medical professionals. In summary, PISF presents a feasible and cost-effective way to significantly boost the widespread adoption of DL in various fast MRI applications.
Main Context
How Can AI Address the Challenges of Multi-scenario Fast MRI with One Model?
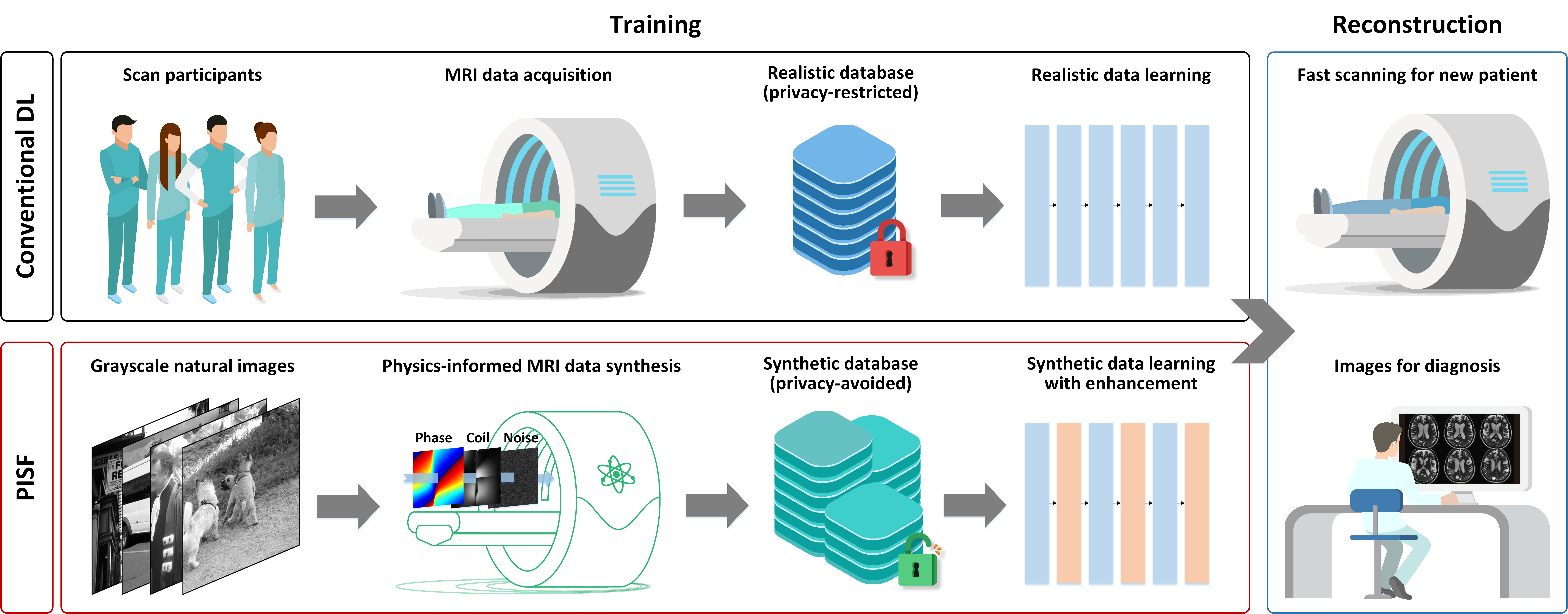
We’ve introduced an innovative physics-informed synthetic data learning framework that tackles the major challenges for AI in fast MRI reconstruction: the lack of realistic training data and performance degradation in multi-scenario applications. Our framework marks a breakthrough by enabling generalizable AI for multi-scenario MRI reconstruction through only one synthetic-data-driven model (Fig. 1).

Fig. 1. The overall concept of the proposed PISF. Top: Conventional DL paradigm for fast MRI reconstruction. It heavily relies on realistic data acquisition to train DL models, which is generally costly and privacy-restricted. Bottom: The proposed PISF enables simplified and scaled-up data curation because numerous synthetic data are generated based on physical forward models. By integrating with enhanced learning techniques, it can perform robust in vivo MRI reconstruction for diagnosis.
By generating large-scale and diverse synthetic data based on MRI physical models (Fig. 2) for training a data-versatile image de-aliasing network, coupled with the data-specific enhancement, we’ve developed a solution that yields MRI reconstructions comparable to or surpassing those of models trained on matched realistic datasets, reducing the reliance on real-world MRI data by up to 96%. With one model, our approach supports high-quality reconstruction under 4 sampling patterns, 5 anatomies, 6 contrasts, 5 vendors, and 7 centers, exhibiting remarkable generalizability. Its adaptability to 2 neuro and 2 cardiovascular patient populations has been validated through evaluations by 10 experienced medical professionals (Fig. 3).
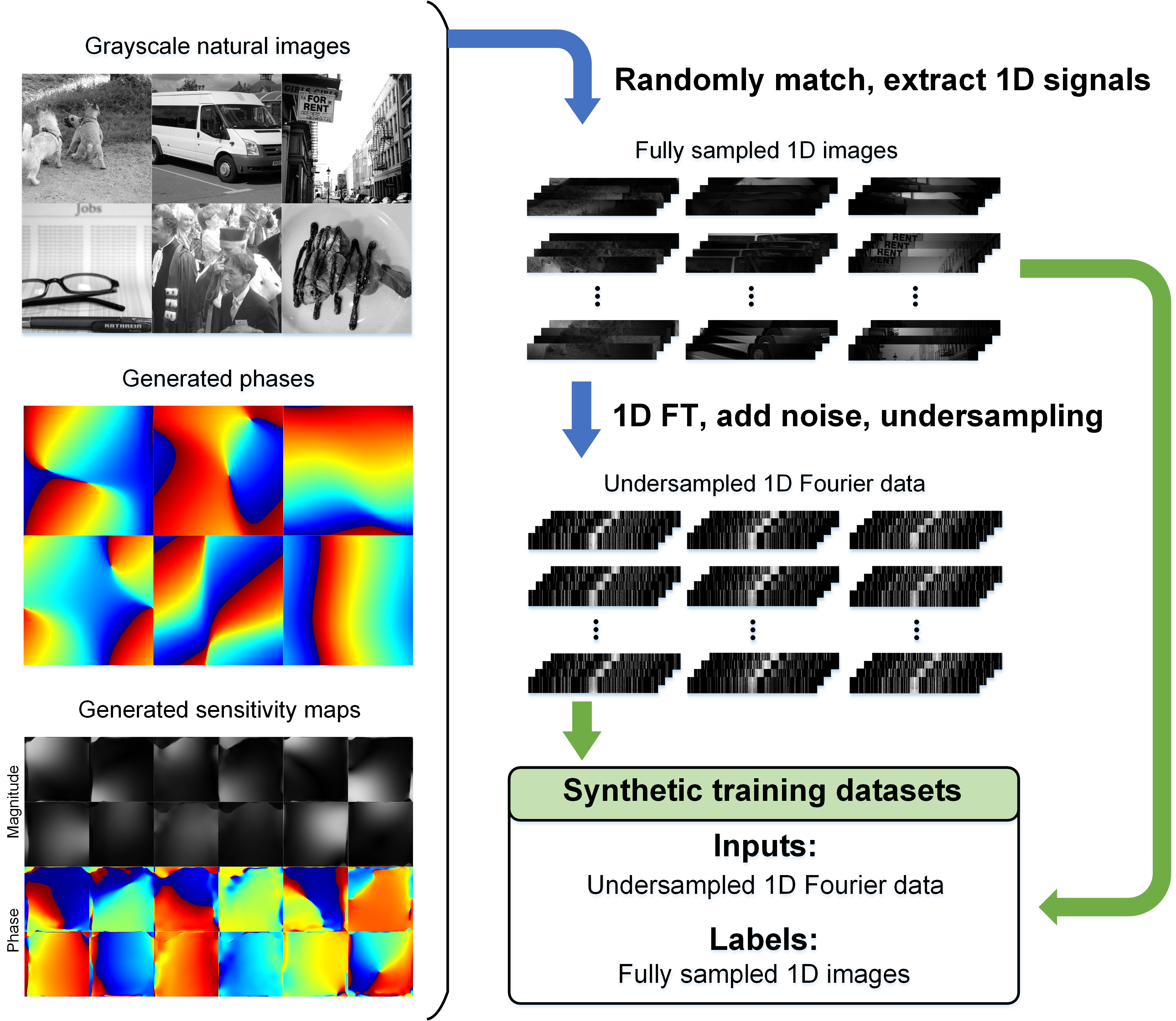
Fig. 2. Flowchart of the physics-informed synthetic data generation.
It offers a feasible and cost-effective way to significantly boost the widespread adoption of AI across various fast MRI applications in healthcare, while circumventing the intractable ethical and practical issues associated with human data acquisition.
Key Features:
1) A novel physics-informed synthetic data learning framework for multi-scenario fast MRI.
2) Decreases the reliance of deep learning on large-scale realistic training data, up to 96%.
3) Enables multi-vendor multi-center MRI reconstruction through one trained model.
4) Adaptive to neuro and cardiovascular patients and verified by ten medical professionals.
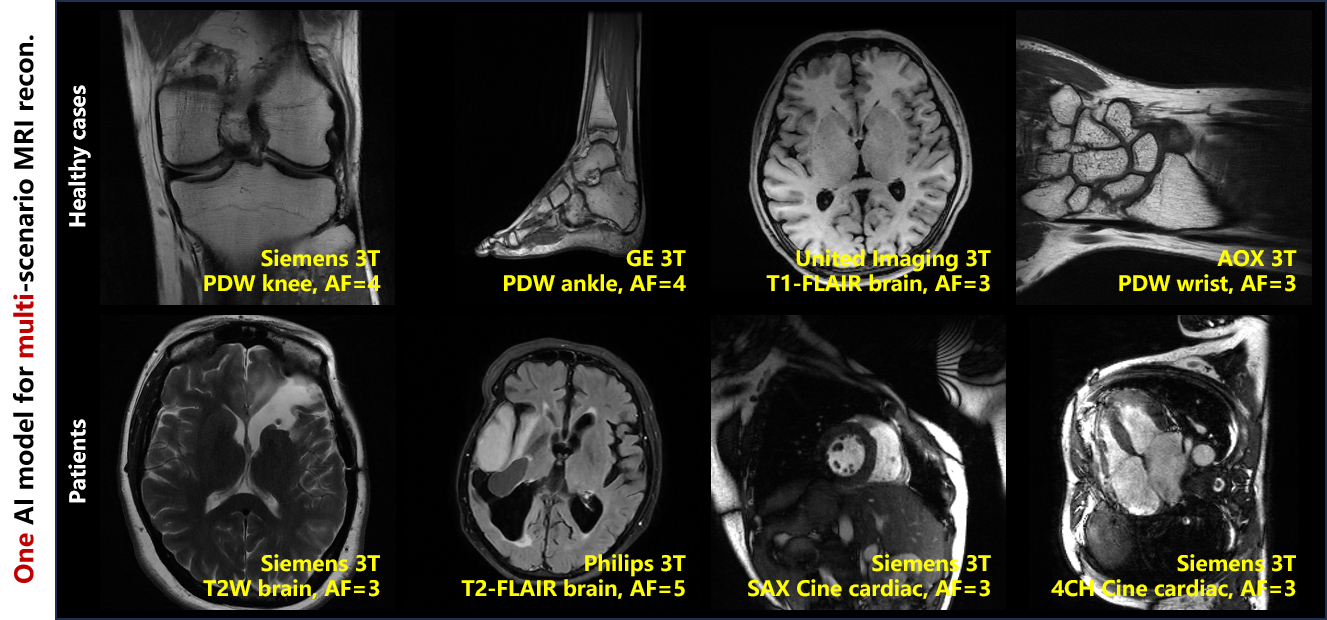
Fig. 3. One PISF model for multi-scenario MRI reconstruction.
Shared Materials
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China under grants 62331021, 62122064, and 62371410, Natural Science Foundation of Fujian Province of China under grants 2023J02005 and 2022J011425, Industry-University Cooperation Projects of the Ministry of Education of China under grant 231107173160805, National Key Research and Development Program of China under grant 2023YFF0714200, President Fund of Xiamen University under grant 20720220063, UKRI Future Leaders Fellowship under grant MR/V023799/1, ERC IMI under grant 101005122, H2020 under grant 952172, MRC under grant MC/PC/21013, the Royal Society under grant IEC\NSFC\211235, UKRI guarantee funding for Horizon Europe MSCA Postdoctoral Fellowships under grant EP/Z002206/1, Shanghai Municipal Science and Technology Major Project under grant 2023SHZD2X02A05, Shanghai Rising-Star Program under grant 24QA2703300, Zhou Yongtang Fund for High Talents Team under grant 0621-Z0332004, Xiamen University Nanqiang Outstanding Talents Program, and China Scholarship Council under grant 202306310177.
References
[1] P. J. Keall et al., "Integrated MRI-guided radiotherapy — Opportunities and challenges," Nature Reviews Clinical Oncology, vol. 19, no. 7, pp. 458-470, 2022.
[2] M. Lustig, D. Donoho, and J. M. Pauly, "Sparse MRI: The application of compressed sensing for rapid MR imaging," Magnetic Resonance in Medicine, vol. 58, no. 6, pp. 1182-1195, 2007.
[3] Z. Liang, "Spatiotemporal imaging with partially separable functions," in IEEE International Symposium on Biomedical Imaging (ISBI), 2007, pp. 988-991.
[4] M. Lustig and J. M. Pauly, "SPIRiT: Iterative self-consistent parallel imaging reconstruction from arbitrary k-space," Magnetic Resonance in Medicine, vol. 64, no. 2, pp. 457-471, 2010.
[5] J. P. Haldar, "Low-rank modeling of local k-space neighborhoods (LORAKS) for constrained MRI," IEEE Transactions on Medical Imaging, vol. 33, no. 3, pp. 668-681, 2014.
[6] Q. Yang, Z. Wang, K. Guo, C. Cai, and X. Qu, "Physics-driven synthetic data learning for biomedical magnetic resonance: The imaging physics-based data synthesis paradigm for artificial intelligence," IEEE Signal Processing Magazine, vol. 40, no. 2, pp. 129-140, 2023.
[7] H. K. Aggarwal, M. P. Mani, and M. Jacob, "MoDL: Model-based deep learning architecture for inverse problems," IEEE Transactions on Medical Imaging, vol. 38, no. 2, pp. 394-405, 2019.
[8] T. Eo, H. Shin, Y. Jun, T. Kim, and D. Hwang, "Accelerating Cartesian MRI by domain-transform manifold learning in phase-encoding direction," Medical Image Analysis, vol. 63, p. 101689, 2020.
[9] A. Pramanik, H. Aggarwal, and M. Jacob, "Deep generalization of structured low-rank algorithms (Deep-SLR)," IEEE Transactions on Medical Imaging, vol. 39, no. 12, pp. 4186-4197, 2020.
[10] J. Zbontar et al., "fastMRI: An open dataset and benchmarks for accelerated MRI," arXiv: 1811.08839, 2019.
[11] V. Antun, F. Renna, C. Poon, B. Adcock, and A. C. Hansen, "On instabilities of deep learning in image reconstruction and the potential costs of AI," Proceedings of the National Academy of Sciences, vol. 117, no. 48, p. 30088, 2020.
[12] Z. Wang et al., "One-dimensional deep low-rank and sparse network for accelerated MRI," IEEE Transactions on Medical Imaging, vol. 42, no. 1, pp. 79-90, 2023.
[13] G. E. Karniadakis, I. G. Kevrekidis, L. Lu, P. Perdikaris, S. Wang, and L. Yang, "Physics-informed machine learning," Nature Reviews Physics, vol. 3, no. 6, pp. 422-440, 2021.
[14] R. J. Chen, M. Y. Lu, T. Y. Chen, D. F. K. Williamson, and F. Mahmood, "Synthetic data in machine learning for medicine and healthcare," Nature Biomedical Engineering, vol. 5, no. 6, pp. 493-497, 2021.
[15] X. Zhang et al., "A guaranteed convergence analysis for the projected fast iterative soft-thresholding algorithm in parallel MRI," Medical Image Analysis, vol. 69, p. 101987, 2021.
[16] Z. Wang et al., "A sparse model-inspired deep thresholding network for exponential signal reconstruction—Application in fast biological spectroscopy," IEEE Transactions on Neural Networks and Learning Systems, vol. 34, no. 10, pp. 7578-7592, 2023.
[17] T. Zhang, J. M. Pauly, S. S. Vasanawala, and M. Lustig, "Coil compression for accelerated imaging with Cartesian sampling," Magnetic Resonance in Medicine, vol. 69, no. 2, pp. 571-582, 2013.
[18] Y. Zhou et al., "CloudBrain-ReconAI: A cloud computing platform for MRI reconstruction and radiologists' image quality evaluation," IEEE Transactions on Cloud Computing, vol. 12, no. 4, pp. 1359-1371, 2024.
[19] J. P. Haldar and J. Zhuo, "P-LORAKS: Low-rank modeling of local k-space neighborhoods with parallel imaging data," Magnetic Resonance in Medicine, vol. 75, no. 4, pp. 1499-1514, 2016.
[20] A. Sriram et al., "End-to-end variational networks for accelerated MRI reconstruction," in Medical Image Computing and Computer Assisted Intervention (MICCAI), Cham, 2020, pp. 64-73.
[21] O. Jaubert et al., "Training deep learning based dynamic MR image reconstruction using open-source natural videos," Scientific Reports, vol. 14, p. 11774, 2024.
[22] B. Yaman, S. A. H. Hosseini, and M. Akcakaya, "Zero-shot self-supervised learning for MRI reconstruction," in International Conference on Learning Representations (ICLR), 2022, pp. 1-16.
[23] Z. Wang et al., "CMRxRecon2024: A multimodality, multiview k-space dataset boosting universal machine learning for accelerated cardiac MRI," Radiology: Artificial Intelligence, vol. 7, no. 2, p. e240443, 2025.