A Paired Phase and Magnitude Reconstruction for Advanced Diffusion-Weighted Imaging (中文,English)
Chen Qian1, Zi Wang1, Xinlin Zhang1,Boxuan Shi1, Boyu Jiang2, Ran Tao2, Jing Li3, Yuwei Ge3, Taishan Kang4, Jianzhong Lin4, Di Guo5, Xiaobo Qu1*
1 Department of Electronic Science, Biomedical Intelligent Cloud R&D Center,
Fujian Provincial Key Laboratory of Plasma and Magnetic Resonance, National Institute for Data Science in Health
and Medicine, Xiamen University, Xiamen 361005, China.
2 United Imaging Healthcare, Shanghai 201807, China.
3 Xingaoyi Medical Equipment Company Limited, Yuyao 315400, China.
4 Department of Radiology, Zhongshan Hospital of Xiamen University, School of Medicine, Xiamen
University, Xiamen 361006, China.
5 Di Guo is with the School of Computer and Information Engineering, Xiamen University of Technology,
Xiamen 361024, China.
Citation
Chen Qian, Zi Wang, Xinlin Zhang, Boxuan Shi, Boyu Jiang, Ran Tao, Jing Li, Yuwei Ge, Taishan Kang, Jianzhong Lin, Di Guo, and Xiaobo Qu, A paired phase and magnitude reconstruction for advanced diffusion-weighted imaging, IEEE Transactions on Biomedical Engineering, DOI: 10.1109/TBME.2023.3288031, 2023.
Background
Diffusion weighted imaging (DWI) is widely used in brain function research [1] and clinical diagnosis [2]. Single-shot echo planar imaging based DWI suffer from low resolution and distortions [3], which are difficult to meet the needs of clinical applications and scientific researches. Multi-shot interleaved echo planar imaging can obtain DWI with improved spatial resolution and less distortions, but its reconstruction will be interfered by the motion phases between shots [4], resulting in serious motion artifacts.
Method and results
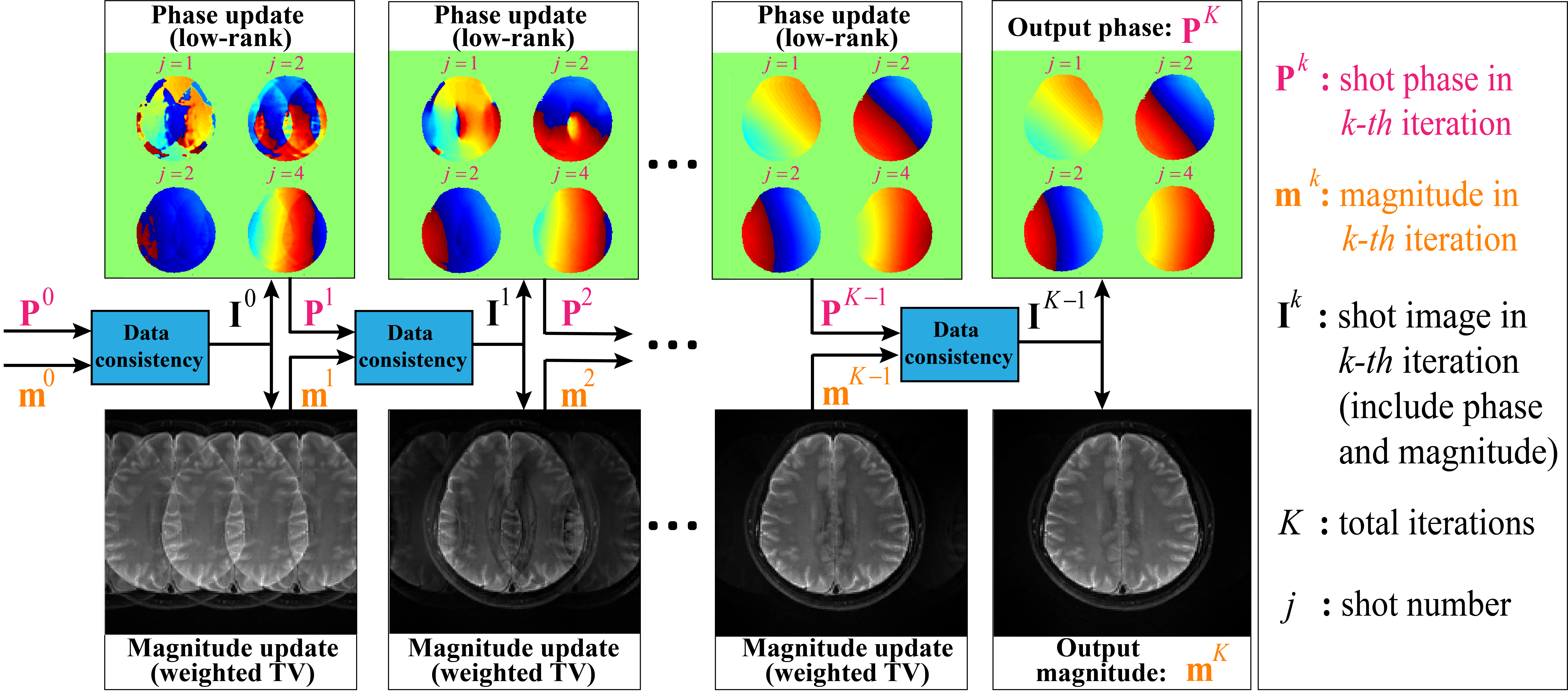
An iteratively joint estimation model with paired phase and magnitude priors is proposed to regularize the reconstruction (PAIR). The former prior is low-rankness in the k-space domain [5]. The latter explores similar edges among multi-b-value and multi-direction DWI with weighted total variation in the image domain. The weighted total variation (TV) transfers edge information from the high SNR images (b-value=0) to DWI reconstructions, achieving simultaneously noise suppression and image edges preservation. The paired shot phase (P) and magnitude (m) priors is incorporated to regularize the updates of shot phase and magnitude, respectively. The whole process is summarized in Fig. 1.

Fig. 1. Iteratively updated shot phase and magnitude images in
PAIR. Low-rank and weighted total variation are used as a pair of complementary priors to facilitate phase
and magnitude image reconstruction. Note: TV is short for total variation.
Compared with PAIR with TV (equivalent to the case where the weights are all 1), PAIR with weighted TV
could similarly suppress noise in the smooth region (white arrows in Fig. 2), and preserve texture
details (blue arrows in Fig. 2).
Fig. 2. Reconstructions by PAIR with different TV. The data
is 4-shot, 17-channel, in-plane resolution 1.0×1.0 mm2, b-value 1000 s/mm2 DWI. (a) is the reference
image m0 (b-value=0). (b)-(d) are results of PAIR with wTV ( ), PAIR with wTV ( ), and PAIR with TV.
The up, middle and down row of (c)-(e) are reconstruction results, corresponding zoom in regions,
and the weights calculated from m0. Note: weighted TV and TV have the same regularization
parameter.
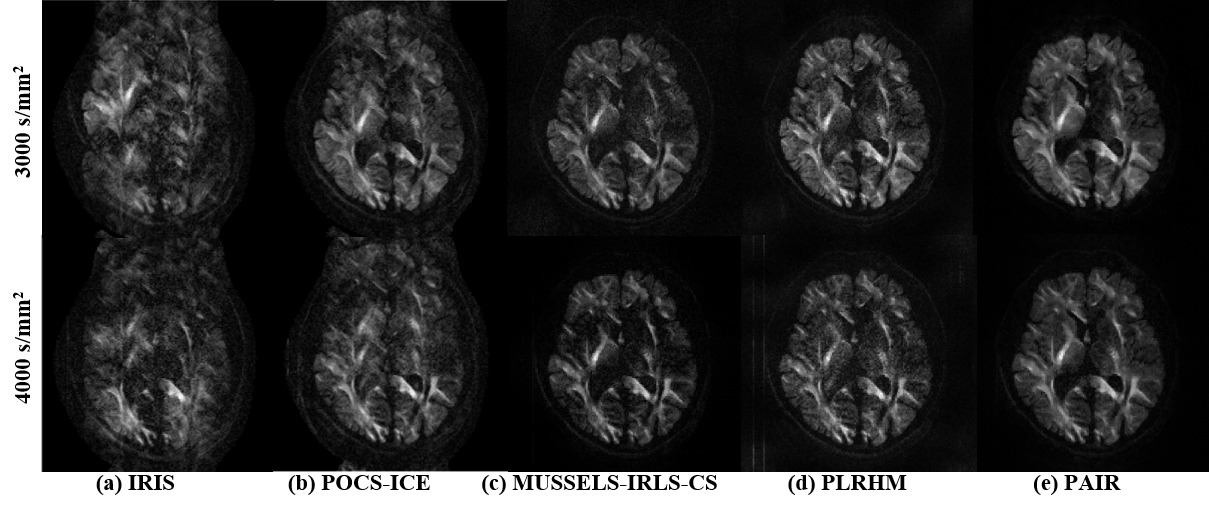
The ultra-high b-values DWI data (3000 and 4000 s/mm2) have a significantly low SNR, which poses a
severe challenge for reconstruction. The proposed PAIR outperforms other methods [6-9] on much better
tolerance to artifacts and noise (Fig. 3(e)).
Fig. 3. Reconstructions of ultra-high b-values eight-shot
data, the resolution is 1.5*1.5 mm2.
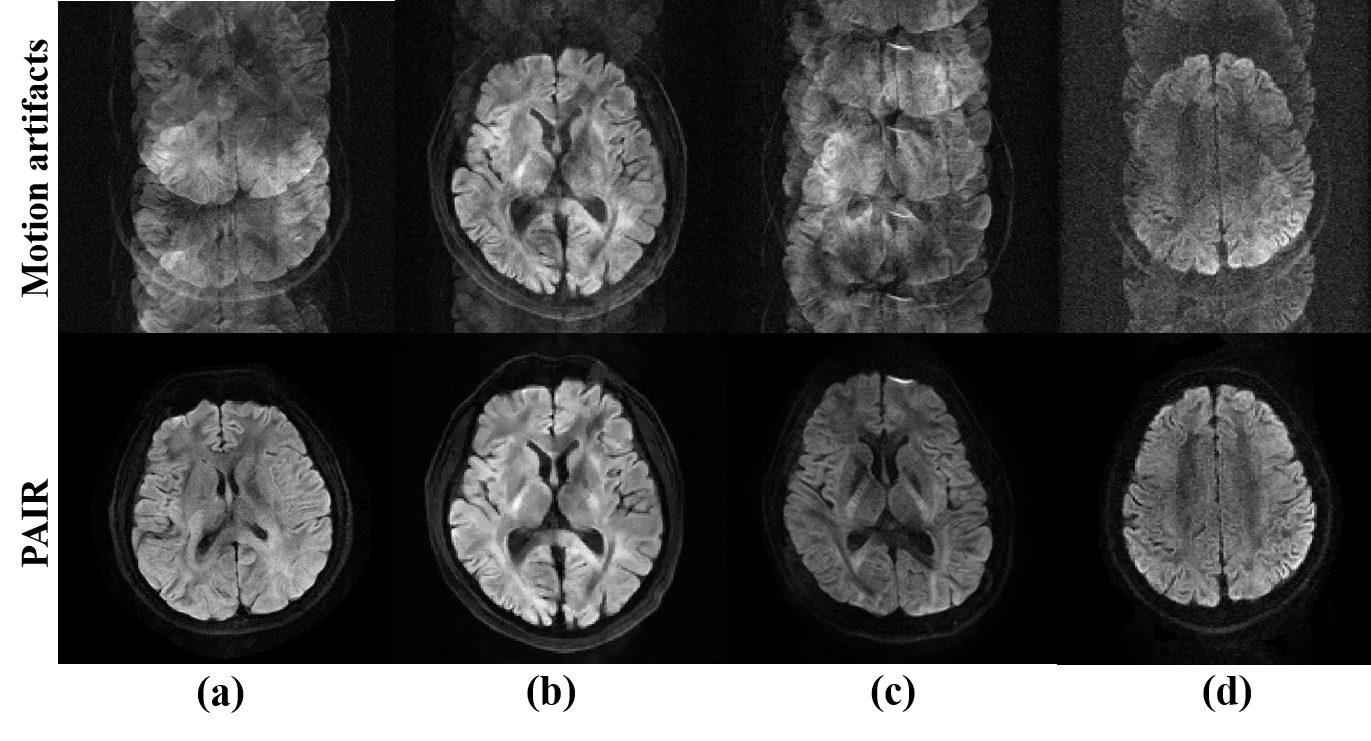
We further test the performance of PAIR on diverse DWI data acquired by scanners from 3 vendors in 4
centers (Fig. 4). PAIR shows robust performance on the above multi-vendor multi-center data, and the
motion artifacts could be removed well (Fig. 4).
Fig. 4. PAIR reconstructions on the multi-center
multi-vendor in vivo data. (a) is from Dataset I: Philips 3.0T scanner in Beijing, China. 8-shot,
8-channel, b-value 800 s/mm2, in-plane resolution 1.0×1.0 mm2. (b) is from Dataset II: United
Imaging 3.0T scanner in Shanghai, China. 4-shot, 17-channel, b-value 1000 s/mm2, in-plane resolution
1.5×1.5 mm2. (c) is from Dataset III: Philips 3.0T scanner in Xiamen, China. 4-shot, 32-channel,
b-value 1000 s/mm2, in-plane resolution 1.2×1.2 mm2. (d) is from Dataset VI: XinGaoYi 1.5T scanner
in Yuyao, China. 4-shot, 8-channel, b-value 1000 s/mm2.
Code
To make it easier to use PAIR, we have implemented and deployed PAIR on the open-access cloud platform,
CloudBrain-ReconAI [10, 11]. (please visit https://csrc.xmu.edu.cn/CloudBrain.html, test account:
radiologist1, password: radiologist1!).
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China under grants
62122064, 61971361, and 61871341, the Natural Science Foundation of Fujian Province of China under grant
2021J011184, President Fund of Xiamen University under grant 20720220063, and the Xiamen University
Nanqiang Outstanding Talents Program.
The authors thank Yiman Huang, Dicheng Chen for the discussions on the MRI reconstruction; Dr. Hua Guo,
Yi Xiao for their assistances in data acquisition and image analysis; Yu Hu for creating video tutorials
on CloudBrain-ReconAI usage; Dr. Mathews Jacob for sharing the IRLS-MUSSELS-CS code online; The authors
also thank the editors and reviewers for the valuable suggestions.
References
[1] D. K. Jones, “Diffusion MRI”. Oxford University Press, 2010.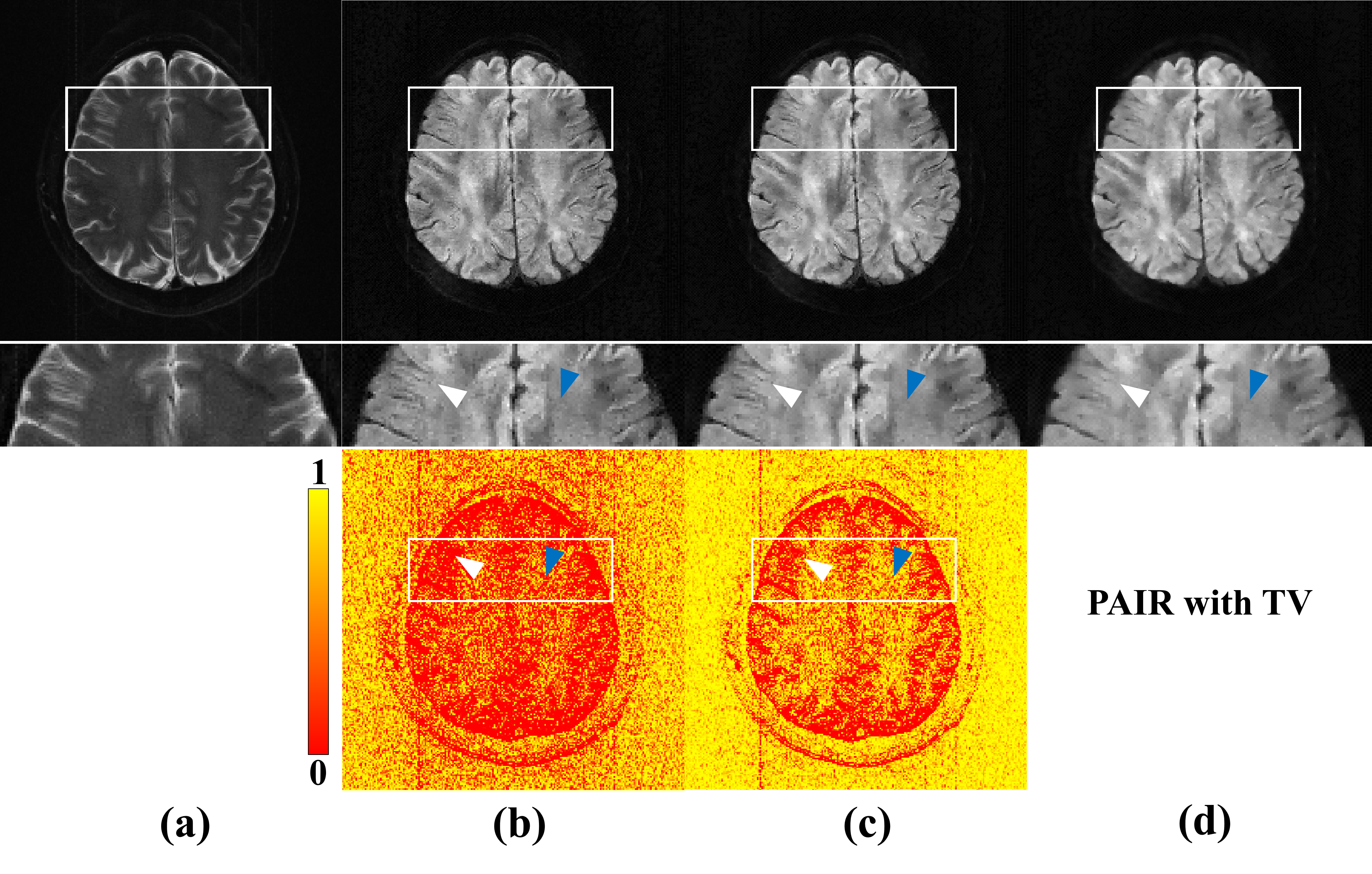
[2] M. G. Lansberg, G. W. Albers, C. Beaulieu, and M. P. Marks, “Comparison of diffusion-weighted MRI
and CT in acute stroke,” Neurology, vol. 54, no. 8, pp. 1557-1561, 2000.
[3] G. C. Baxter, A. J. Patterson, R. Woitek, I. Allajbeu, M. J. Graves, and F. Gilbert, “Improving the
image quality of DWI in breast cancer: comparison of multi-shot DWI using multiplexed sensitivity
encoding to conventional single-shot echo-planar imaging DWI,” The British Journal of Radiology, vol.
94, no. 1119, 20200427, 2021.
[4] A. W. Anderson and J. C. Gore, “Analysis and correction of motion artifacts in diffusion weighted
imaging,” Magnetic Resonance in Medicine, vol. 32, no. 3, pp. 379-87, 1994.
[5] J. P. Haldar, “Low-rank modeling of local k-space neighborhoods (LORAKS) for constrained MRI,” IEEE
Transaction on Medical Imaging, vol. 33, no. 3, pp. 668-681, 2013.
[6] H. Guo, X. Ma, Z. Zhang, B. Zhang, C. Yuan, and F. Huang, “POCS‐enhanced inherent correction of
motion‐induced phase errors (POCS‐ICE) for high‐resolution multishot diffusion MRI,” Magnetic Resonance
in Medicine, vol. 75, no. 1, pp. 169-180, 2016.
[7] M. Mani, M. Jacob, D. Kelley, and V. Magnotta, “Multi‐shot sensitivity‐encoded diffusion data
recovery using structured low‐rank matrix completion (MUSSELS),” Magnetic Resonance in Medicine, vol.
78, no. 2, pp. 494-507, 2017.
[8] M. Mani, H. K. Aggarwal, V. Magnotta, and M. Jacob, “Improved MUSSELS reconstruction for
high-resolution multi-shot diffusion weighted imaging,” Magnetic Resonance in Medicine, vol. 83, no. 6,
pp. 2253-2263, 2020.
[9] Y. Huang et al., “Phase-constrained reconstruction of high-resolution multi-shot diffusion weighted
image,” Journal of Magnetic Resonance, vol. 312, 106690, 2020.
[10] Q. Yang, Z. Wang, K. Guo, C. Cai, and X. Qu, “Physics-driven synthetic data learning for biomedical
magnetic resonance: The imaging physics based data synthesis paradigm for artificial intelligence,” IEEE
Signal Processing Magazine, vol. 40, no. 2, pp. 129-140, 2023.
[11] Y. Zhou et al., “XCloud-pFISTA: A medical intelligence cloud for accelerated MRI,” in 43rd Annual
International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 2021, pp.
3289-3292.